Rust Removal using Electrolysis
What you need:
- A non-conducting container - a large plastic bucket works really well.
- Battery charger - big is better, however even one able to produce 6 to 10 amps should do.
- Sacrificial electrodes. Concrete reinforcing rod works well (rebar) cut into lengths Do not use stainless steel! The results are a health hazard and illegal.
- Arm and Hammer LAUNDRY soda, also called washing soda. (see below for details) Wire and/or cables for connecting electrodes together. Water Small lengths of small chain (used to suspend the rusty parts in solution) or some other means to suspend the part to clean into the solution.
The Setup:
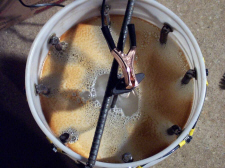
Using a plastic, or non-conductive bucket (not metal), mix a solution of 5 gallons water to 1/3 to 1/2 cup laundry soda. Mix well so all soda is dissolved. Adding more soda will not make it go faster. Do not try to use other salts. You won't get better results and dangerous effects may occur. Caustic soda, for example, is far too corrosive. Solutions of ordinary table salt can generate chlorine gas (toxic) at the positive electrode (anode). Clean the electrodes so they aren't too rusty - especially at the top ends - they need to make good electrical contact with your wire or cable AND with the water. Take them to a wire wheel and give them just a real quick going over. Place electrodes in bucket around sides, so the clean, rust free ends stick up above the bucket. Use clamps or some means to hold them in place around the perimeter of the inside of the bucket or container so that they cannot move freely or fall into center of bucket. The electrodes must not touch the part(s) to be cleaned, which will be suspended in center of bucket. Whatever you use, it shouldn't be copper, and will get a bit messy if it gets into your cleaning solution. Tie the electrodes together with wire or cables. All electrodes need to be tied together, -electrically speaking, -one big electrode. This will become the grid. Since the cleaning process is somewhat line of sight it's best to surround the part to be cleaned to some extent with the electrodes.
Suspend part to be cleaned into bucket so it hangs in the middle, not touching bottom, and not touching electrodes. I place a piece of rebar across top of bucket (see photo below) and bolt a small piece of chain to my part to be cleaned, and clamp the chain on the rod so that the chain hangs from the rod, and suspends the part into solution below. The part to clean then becomes the "cathode". Attach battery charger - place NEGATIVE LEAD (this is critical!!) on the piece that is to be cleaned. Attach POSITIVE, or RED lead of charger, to electrode grid formed when you placed electrodes, or rods, into bucket and tied them all together. Make sure electrodes and part to be cleaned are not touching each other. Do not get this backwards! If you do, you'll use metal from your part to de-rust your electrodes instead of the other way around. Now turn on the battery charger. If the current is too high on the battery chargers current meter there are a number of things you can do to reduce it,
- Increase the distance between the part and the anode
- Dilute the solution by adding more water
- If you have a 6/12 volt charger set it to the 6 volt setting.
Within seconds, you should see a lot of tiny bubbles rising from the part suspended in the mixture. Do not do this inside, or in a closed area those bubbles are the component parts of water - H2O - hydrogen and oxygen. The hydrogen will burn explosively …Remember the government made bombs out of hydrogen.
The rust and gunk will bubble up to the top and form a gunky layer. More gunk will form on the electrodes after some amount of use, they will need to be cleaned and/or replaced - the electrodes give up metal over time. That's why re-bar is such a nice choice - it's cheap and easy to get in pre-cut lengths. Now you just have to wait. The time required to clean a part will depend on many variables:
- size of the part
- current used
- how badly rusted the part is
The process is self-halting; when there is no more rust to remove, the reaction stops. This is handy because you don't have to monitor it, and because you can do large parts where they are not totally submersed at one time without worrying about lines in the final part. If necessary it is acceptable to leave the operation on overnight so long as it is not in an enclosed space (see the safety precautions below). You may have to move the piece occasionally for better cleaning as the best cleaning is done on the part that is in direct view of the anode (line of sight). If a piece is too large to fit in the bath you will obviously have to rotate it at some point. It may also be necessary to take the part out of the bath and clean it with a wire brush to remove some of the now loose scale which will look like a dark sludge.
Once you are done, the part should be dried immediately, the part is very susceptible to surface rust after being removed from the solution. There will be a fine layer of dark grey or black residue on the part that can be easily removed, a grey scrub pad works great, and once it is removed, the part can be primed or painted as needed.
You can pour the waste solution on the lawn and it won't hurt it. Do watch out for ornamental shrubs, which may not like iron rich soil.
How large an item can you clean? Well, it's up to your imagination, your budget - because it takes water, your time and patience. On a very large scale using a tank made of plywood and lined with plastic, a DC welder for power supply and hundreds of gallons of water. You will need to use more electrodes with larger parts and a larger "tank".
Washing soda
Washing soda should be in the laundry section of your grocery store. It comes in a yellow box, made by Arm & Hammer, but it's NOT baking soda. If you're interested, washing soda is Sodium Carbonate (Na2CO3), baking soda is Sodium Bicarbonate (NaHCO3), and Borax is Sodium Tetraborate Decahydrate (Na2B4O7*10H2O), all different chemical compounds.
Safety Precautions:
- Make sure no spills can get to the battery charger. (Electrocution potential)
- The leads from the charger are relatively safe, but you may still get a bit of a shock if you put your hands in the solution or touch the electrodes while the charger is running.
- Turn off the current before making adjustments to the setup. Just as a "spark" can cause a charging battery to explode in your face, this process produces similar gases because this process splits water into hydrogen gas (at the negative electrode) and oxygen (at the positive electrode).
- Hydrogen will burn explosively if ignited. All flames, cigarettes, torches, etc. must be removed from the area, and sparks caused by touching the leads together must be avoided. The work should be performed outside or in a well ventilated area to remove these gases safely.
- Washing soda solutions are alkaline and will irritate the skin and eyes. Use eye protection and gloves. Immediately wash off any solution spilled or splashed onto your body.
Why you should not use stainless steel:
Many people using the electrolysis method for rust reduction swear by stainless steel, stating (incorrectly) that it's not consumed, stays clean and seems safe. Stainless steel is indeed consumed when used in the electrolysis process, although slowly. The main problem with using it is the hazardous waste it produces. Stainless steel contains chromium. The electrodes, and thus the chromium consumed, and you end up with poisonous chromates in your electrolyte. Dumping these on the ground or down the drain is illegal. The compounds can cause severe skin problems and ultimately, cancer. Hexavalent chromate is poisonous. These compounds are not excused from hazardous waste regulations where household wastes are. These compounds are bad enough that government regulations mandate elimination of hexavalent chromate by 2007 for corrosion protection.
Does your electrolyte turn yellow? That's a sign of chromates.
If you have been using stainless steel for the anodes (positive electrodes), wear rubber gloves when working with or near the liquids. If you need to dispose of it, allow it to evaporate into powders and dispose of the powders in sealed containers during your local "hazardous waste clean-up days".
Best bet - don't use stainless steel no matter how tempting it is.